Antimicrobials on the farm: The need for responsible use to tackle AMR
Clinical Connections – Autumn 2018
Andrew Rycroft, Professor of Clinical and Veterinary Microbiology
At last, we seem to have grasped the idea that antimicrobial resistance (AMR) is something that can affect us all.
The knowledge that bacteria can resist the effects of antibiotics isn’t new. Penicillin resistance was detected shortly after its first clinical use. Resistance existed all along – it was found in microbes stored away even before Alexander Fleming discovered penicillin at St Mary’s Hospital.
What has changed in recent years is the realisation that we need to reduce our antimicrobial drug use to limit resistance development and preserve as much of their value as possible.
Antimicrobials are regularly misused. They have been considered the easy option, without apparent side-effects. When I have enquired of veterinary students’ experiences on farms, working with pigs. “Any disease?”, I would probe. “Diarrhoea” would be a regular answer. “And what did the farmer do?” “Oh, he just gave them all an injection of Baytril.” would be a common response.
Of course Baytril is enrofloxacin. This superb drug, introduced in 1992, has very low MICs for many important pathogens and very low toxicity.
So why were farmers administering a fluoroquinolone? Why was it available to them? Was this a responsible use of this prescription only medicine? Why were the animals given an antibacterial drug when there was no evidence of bacterial infection (many things cause diarrhoea in pigs)? In reality, many farms have dozens of bottles of antibiotic available for farmers to “treat” ailments as they see fit. This situation has developed though farmers’ need to act quickly when the welfare (and value) of animals is threatened. Advice from the vet can be avoided.
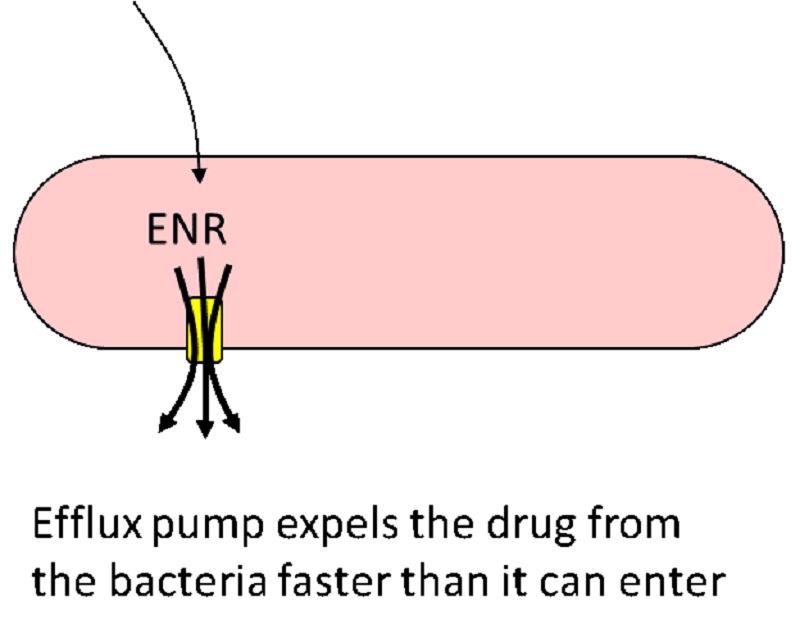
How can this lead to antibiotic resistance that could affect humans? Surely the bacteria causing human infections are quite different from those causing animal disease? It is a common misconception that when an antimicrobial drug is used it targets only the causative pathogen. Animals carry a wide range of bacteria on the skin, the gut and the upper respiratory tract. They excrete active drug into the environment, in faeces and urine. All these microorganisms may be exposed to low concentrations of a drug, and resistant bacteria arise through genetic exchange and selection among the immense numbers of bacteria in the environment. In the case of enrofloxacin, resistance happens when specific mutations occur in the DNA encoding the topoisomerases needed for unwinding the bacterial DNA, and when the genes encoding common membrane proteins (bacterial efflux pumps) are altered to be more capable of expelling enrofloxacin.
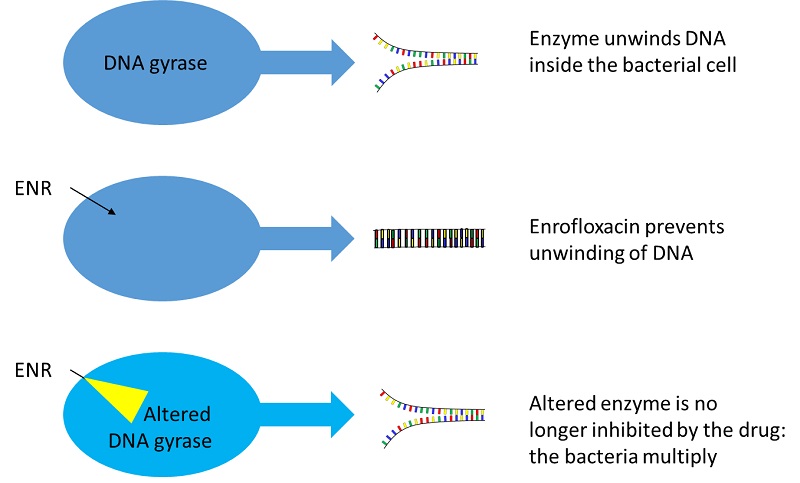
These mutations occur all the time – we never know about them. But when antibiotic is present, those bacteria with the mutations have a substantial advantage over others. Such genes can be transferred from common harmless skin or gut bacteria into pathogens.
Some advocate a total ban on antimicrobials in animals, particularly farm animals. But a total ban would have huge welfare implications for farm animals: infections would go untreated. Another view is that critically important antimicrobials: 3rd generation cephalosporins, fluoroquinolones, glycopeptides and carbapenems, should be reclassified as controlled drugs (in line with opiates and barbiturates) restricting their use to administration by a veterinary surgeon. In March 2016, the European Parliament backed proposals to ban the prophylactic use of antibiotics in animals. Clearly, there is a conflict between public health, the farmer’s economic imperatives and the welfare of animals. This is not easily resolved.
So, what can we do? What should we be doing? It is important that the veterinary profession is seen to be promoting responsible attitudes. The 2016 O’Neill Report recommended interventions to reduce the demand for antimicrobials. Practical guidance has been offered by BVA, BSAVA and RUMA. There are encouraging signs of action. A number of initiatives in more progressive farm animal veterinary practices are showing very positive results. The quantity of antimicrobials in pigs and poultry production has already fallen below the targets expected by DEFRA (multispecies average of 50 mg/kg by 2018).
The opportunity exists for this to be extended as the post-Brexit policy for agriculture is developed. Radical changes in the stewardship of important antimicrobial drugs should now be a priority and could contribute significantly to UK public and animal health.
