Malassezia Spp. is a Component of the Duodenal Mycobiota of Dogs With Chronic Enteropathy
Clinical Connections – Summer 2022
Ross Bond, Professor of Veterinary Dermatology, and Aarti Kathrani, Senior Lecturer in Small Animal Internal Medicine
The veterinary profession is increasingly aware of the role intestinal dysbiosis may play in a range of disorders in body systems other than the gastrointestinal tract. Attention has tended to focus on bacterial dysbiosis of the gut but the role of intestinal fungal dysbiosis in the overall process is something we are exploring as well.
The RVC’s Dermatology Service has been investigating colonisation and infection of canine and feline skin by Malassezia yeasts since 1992, when Professor Bond undertook a PhD research programme funded by Clinical Studies Trust Fund, the predecessor of BSAVA Petsavers. During that time, Mrs Anne Lamport, the then dermatology research technician, noted the occasional isolation of M. pachydermatis from duodenal juice samples obtained during endoscopy of dogs with enteropathy. Although this was not followed up, there were no reports of the isolation of this genus from the intestine of mammals at that time. The usual ecological niche for this genus of lipophilic yeasts is the stratum corneum of a variety of mammals and birds.
The mammalian intestinal microbiota represents a highly complex ecosystem of bacteria, fungi, viruses and protozoa that protects the host against potential enteric pathogens. Microbial dysbiosis is often marked in various enteropathies; investigations have primarily focused on modifications in bacterial populations, whereas fungi have received much less attention. More recently however, considerable scientific interest in enteric Malassezia has developed following recognition of significant fungal dysbiosis in humans with Crohn’s disease, a subtype of human inflammatory bowel disease.
Furthermore, Malassezia spp. dysbiosis has been implicated in tumorigenesis of human and mouse pancreatic ductal adenocarcinomata. In mouse models, fungal ablation was tumour-protective, whereas Malassezia re-population accelerated oncogenesis. These observations, and our own previous unreported observations from the 1990s, prompted Professor Bond and Dr Aarti Kathrani to devise a study with the aim of defining the duodenal mycobiota of dogs undergoing endoscopy for the investigation of chronic enteropathy. The study was done in collaboration with Bart Theelen of the Westerdijk Fungal Biodiversity Institute, Utrecht, and kindly funded by BSAVA Petsavers.
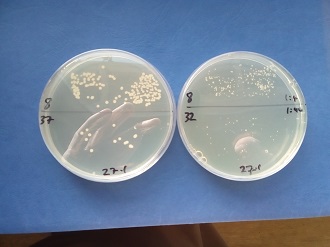
The study was approved by the RVC’s Clinical Research Ethical Review Board and informed consent was obtained from dog owners prior to sampling. Quantitative microbial culturing was performed on duodenal juice aspirated from dogs with suspected chronic enteropathy during routine upper gastrointestinal endoscopy. Samples were cultured on Sabouraud dextrose agar and modified Dixon agar for 14 days. Isolates were identified phenotypically, and by matrix-assisted laser desorption / ionisation time-of-flight (MALDI-TOF) and internal transcribed spacer (ITS) sequencing.
A total of 45 dogs were recruited with chronic inflammatory enteropathy (n=38), granulomatous colitis (n=2), gastric adenocarcinoma (n=2), duodenal small cell lymphoma (n=1) and idiopathic severe gastrointestinal hemorrhage (n=2). Fungi were cultured from 14 dogs: M. pachydermatis was isolated from eight (chronic inflammatory enteropathy [n=7] [along with Candida albicans n=1]; granulomatous colitis [n=1]) and M. sympodialis from another (gastric adenocarcinoma). Five dogs with chronic inflammatory enteropathy yielded other yeasts (C. albicans, C. glabrata, Kazachstania slooffiae, K. telluris, Pichia kudriavzevii [syn. C. krusei]).
Yeast organisms were not observed in any of the biopsy specimens by histopathological examination, despite staining with Gomori’s methenamine silver (which stains fungal cell walls black). In cytological samples stained with Diff-Quik (n=45) and examined using light microscopy, yeasts / fungi were never observed. However, in cytological specimens also stained with calcofluor white and examined using fluorescence microscopy (n=19), fungi were observed only in one case. In this case, a single example of a yeast with a germ tube was noted (Kazachstania slooffiae isolated).
Dogs with yeast growth had significantly higher serum vitamin B12, canine chronic enteropathy clinical activity index and dermatological signs at the time of yeast isolation versus those with no growth (p=0.003, p=0.034 and p=0.043, respectively).
To our knowledge, this is the first report of isolation of M. pachydermatis, and M. sympodialis and Kazachstania spp. from the canine duodenum. Future studies will help to determine the effect of yeast colonization on treatment response and prognosis – and thus whether any benefit could be gained from anti-fungal therapy in these dogs.
We are most grateful to the owners of the dogs for agreeing to participate in the study, and to the members of the Internal Medicine and Endoscopy teams who facilitated the investigation.
