
PK/PD informed clinical breakpoint determination for colistin in chicken to limit emergence of resistance and improve One Health antimicrobial sustainability
This project aims to evaluate the impact of colistin use on antimicrobial resistance and rationalise dosing through a combination of in vivo pharmacokinetic (PK) dose studies, in vitro pharmacodynamic (PD) and antimicrobial susceptibility testing, and advanced in silico PK/PD modelling.

Challenge
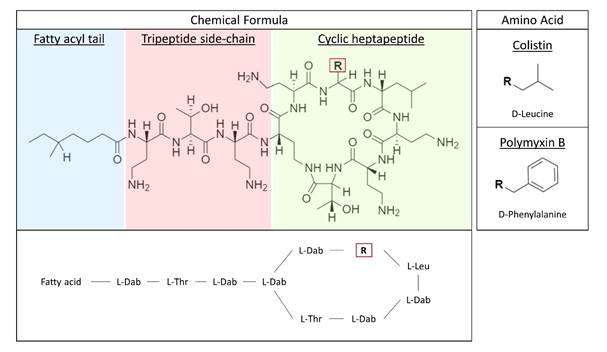
Colistin (polymyxin E) is categorised as a highest priority critically important antimicrobial by the World Health Organisation (WHO) and a veterinary critically important antimicrobial by the World Organisation for Animal Health (OIE), indicating the essential nature of this antimicrobial in both human and veterinary medicine.
Antimicrobial resistance (AMR) is a leading cause of global death, with 1.27 million deaths directly attributable to AMR in 2019 (Murray et al., 2022). Colistin is a last-line antibiotic to treat multidrug resistant gram-negative infections in humans. In veterinary medicine colistin is used to treat infections caused by Enterobacteriaceae in pigs, poultry, and cattle, such as enterocolitis and colibacillosis. To maintain this antibiotic for human use, with consideration to the recent discovery of mobilised colistin resistance (MCR) and its global prevalence in bacteria from both humans and animals, the European Medicines Agency (EMA) advocated a reduction in colistin sales and usage in animals. The poultry sector is one of the largest global livestock industries, with more than 23 billion chickens worldwide (Mottet and Tempio, 2017), indicating the extent of potential animal welfare issues and production losses associated with the reduction of colistin use, which remains an almost irreplaceable veterinary antimicrobial for intestinal E. coli infections in poultry.
To maintain colistin as an essential antimicrobial for both human and veterinary use, recent and reliable data regarding the pharmacokinetics (PK) and pharmacodynamics (PD) at the clinical dose in poultry, and its impact on the potential selection of resistance is required to inform application and policy.

Solution
A comprehensive understanding of the effects of colistin treatment in broiler chickens will be achieved through:
- in vivo studies of PK at clinical (75 000 IU/kg/day) and supra-clinical doses (100 000 and 150 000 IU/kg/day) in drinking water and oral gavage (75 000 and 150 000 IU/kg) as measured in the small intestine via UHPLC-MS/MS;
- enumeration of E. coli pre-, during- and post-treatment with associated antimicrobial susceptibility testing via minimum inhibitory concentration (MIC) assays and screening of mobilized colistin resistance (mcr) via PCR;
- evaluation of the wider impact on the gut flora using 16s microbiomics;
- determination of the PD of colistin through time-kill analysis against both wild-type susceptible isolates and low-susceptibility isolates (MIC ≥ ECOFF);
- advanced in silico PK PD modelling to determine a clinical breakpoint for E. coli in broiler chickens and predict the impact of alternative dosing regimens.
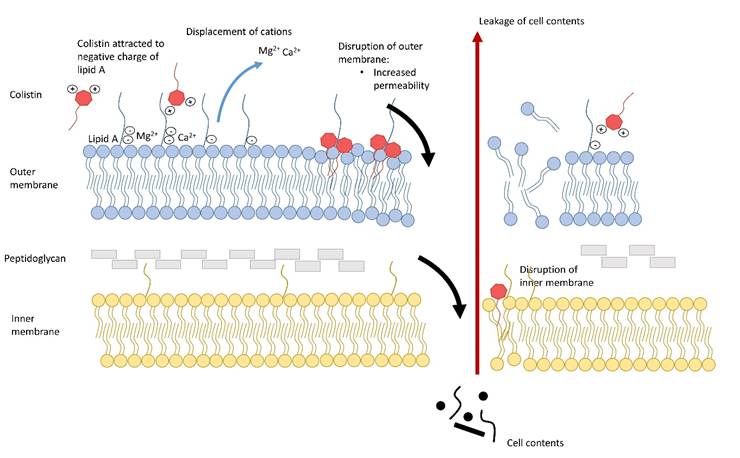
Impact
The continued veterinary use of colistin in livestock, including poultry, remains under intense scrutiny from both veterinary and human sectors. With the introduction of European Parliament Regulation (EU) 2019/6 (from Jan 2022), which outlines the criteria for reservation of antimicrobials only for certain infections in humans, the need for current reliable data surrounding colistin use is critical for determining future policy. The Federation of Veterinarians of Europe (FVE) have directly cited our work to support the ongoing policy discussion regarding colistin use, which has further been acknowledged by the EMA (EMA, 2022), and have subsequently shown further interest in the full thesis. The outcomes of these discussions will have a profound impact on animal welfare, practicing veterinarians, farmers, and the pharmacological industry. Colistin research continues to draw industry interest from companies including VMD livestock pharma, Dopharma, Virbac.
At this time of heightened interest in antimicrobial stewardship, species specific PK/PD data is vital for veterinarians and farmers to ensure they are providing treatment that is both clinically effective and has as minimal an impact on the existing gastrointestinal microbiota as possible. Our data provides a basis for reviewing the current clinical dose and its impact on AMR, with other researchers using the data to explore alternative dosage regimens, and building deterministic models describing the potential spread of resistance in animals and the environment. In conclusion, my results provide critical data regarding colistin PK, PD and AMR to promote prudent antimicrobial use and limit the spread of resistance, at a key point in the policy decision pipeline. This is likely to have long-term and widespread impact from a One Health perspective on an antimicrobial that is critical to both human and veterinary medicine.
Partners
Dopharma
V.M.D./Inovet
Virbac
TransPharm
BBSRC (LIDo)
