How to grow new blood vessels: the zebrafish as a model to study angiogenesis in development and regeneration
Angiogenesis is the growth of new vessels from pre-existing ones. It is an important process during development but also in other physiological and pathological processes in adults.
Updated 7 March 2025
For example, after a heart attack the growth of new vessels is important to restore blood flow back to the damaged cardiac tissue. Similarly, the generation of new vascular networks towards legs and arms is beneficial for patients suffering from peripheral artery disease (PAD) whose arteries became blocked due to the building of atherosclerotic plaque. On the other hand, restricting angiogenesis has been a focus for therapeutic approaches in cancer, with the aim of depriving the tumour of blood flow and therefore hindering its growth.
The zebrafish genome has been sequenced in 2013 and it was found that many genes are conserved structurally and functionally. They have a relatively short generation time and external fertilisation and their transparency allow the easy visualisation of all stages of development. Within two to three days post-fertilisation, all major organs have been laid out, the major axial trunk vessels have been formed and the heart is beating.
At the Royal Veterinary College, we are studying how Neuropilins (which are receptors important for the growth of new blood vessels) regulate angiogenesis by observing the early development of the zebrafish embryo. Using microscopy, we study how the arteries and veins develop in embryos with vessels that been tagged with fluorescent proteins (see image below).
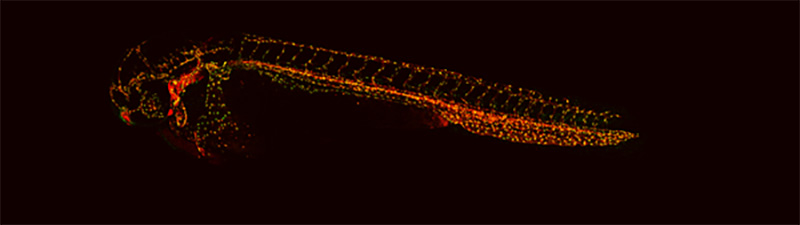
This specific transgenic line is called (tg(fli1a:nEGFP)y7; tg(fli1a:DsRedex)um13) and has blood vessels (arteries and veins) tagged with a red fluorescent protein and green fluorescent nuclei (identified as yellow dots).
We are also investigating the role of angiogenesis in the context of regenerative medicine using the caudal fin regeneration model. Although healthy mammals are able to heal wounds, their ability to regenerate complex tissues is very limited. The zebrafish has the remarkable ability to regenerate many organs and appendages (e.g. brain and heart) and also fins. Because of its accessibility and relatively simple architecture, the zebrafish caudal fin regeneration model has proved to be a useful method for investigating the different stages and cell processes involved in epimorphic regeneration. Moreover, the availability of transgenic fish lines with fluorescent reporters allows the identification of several cell types, for example, endothelial cells and macrophages, known to be key players in the regenerative process. Following amputation, it takes approximately 14 days to regenerate a functional fin completely (see figure below).
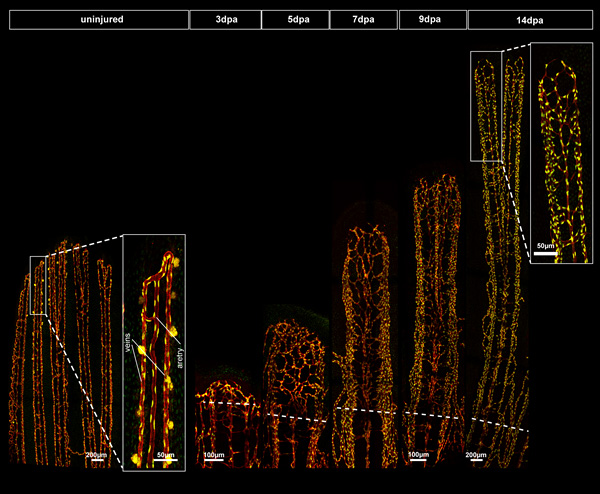
Transgenic fish with red endothelial cells demarking vessels and green nuclei (tg(fli1a:nEGFP)y7; tg(fli1a:DsRedex)um13) were amputated and then imaged on a confocal microscope over 14 days.
The two images on the left show the organisation of the uninjured fin ray vasculature. The high magnification panel shows typical organisation of a central artery flanked by two veins on the outside. From three days onwards after amputation, the blastema (mass of undifferentiated mesenchymal cells) grows and differentiates into the slow proliferating distal blastema and a fast proliferating proximal blastema which progressively switches from the proliferation program to the morphogenesis program.
